Pakistan approves Sinopharm COVID-19 vaccine for emergency use
 0 Comment(s)
0 Comment(s) Print
Print E-mail Xinhua, January 19, 2021
E-mail Xinhua, January 19, 2021
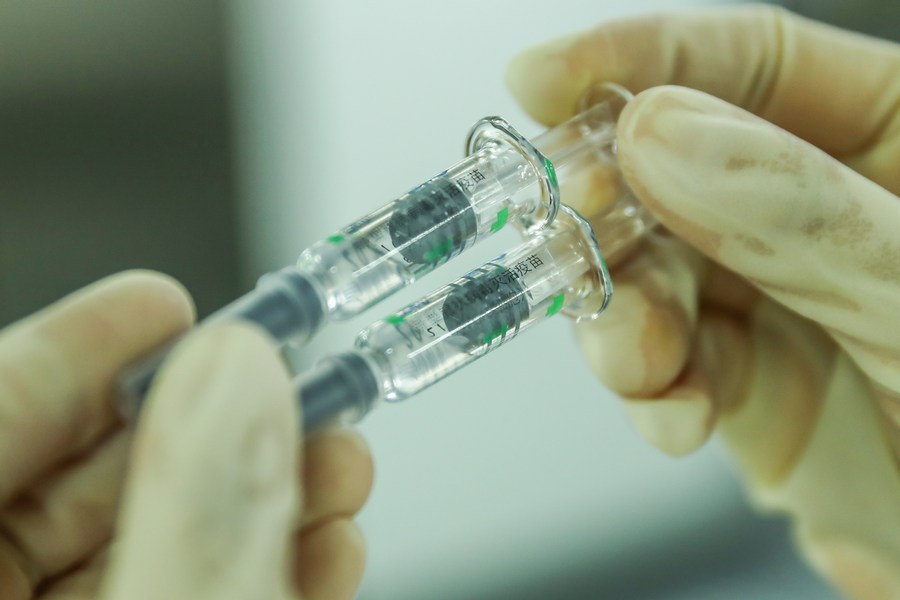
The Drug Regulatory Authority of Pakistan (DRAP) has approved a vaccine by China's Sinopharm for emergency use amid rising cases of COVID-19 in the country.
A handout by DRAP on its official website late Monday read that Sinopharm, one of the two vaccines approved by the authority, has been given emergency use authorization after evaluating its safety and quality.
The authority said the authorization will be reviewed every quarter keeping in view further data regarding safety, efficacy and quality.
A vaccine manufactured by British-Swedish pharmaceutical company AstraZeneca has also been given the authorization earlier, the handout said.
Chaudhry Fawad Hussain, the country's minister for science and technology, told Xinhua earlier that the Sinopharm vaccine has been approved for procurement by a cabinet committee for its safety and affordability.
Pakistan reported 1,920 new coronavirus infections and 46 deaths on Monday, bringing the total number of cases to 521,211 with 10,997 deaths.






Go to Forum >>0 Comment(s)