China launches campaign to ensure medicine safety
![A customer studies the labels on medicine at a drug store in Dandong, Liaoning province.[China Daily] A customer studies the labels on medicine at a drug store in Dandong, Liaoning province.[China Daily]](http://images.china.cn/attachement/jpg/site1007/20110509/0011111fa1560f31737405.jpg) |
|
A customer studies the labels on medicine at a drug store in Dandong, Liaoning province.[China Daily] |
China is set to launch a nationwide probe into the quality of essential drugs in a bid to protect people from problem medication, according to an online notice issued by the State Food and Drug Administration on Friday.
The drugs being looked at are those deemed to satisfy the basic healthcare needs of the majority of the population.
The two-month examination will look closely at how drugs are produced and where their raw materials are sourced, said the notice.
The way traditional Chinese medicine is prepared will also be looked at.
Drug authorities across the mainland will carry out on-site examinations at production facilities and report their findings to the administration before the end of June.
The official statistics show that currently 2,822 companies are producing essential drugs on the mainland.
Through such campaigns, an effective working mechanism that ensures drug safety, particularly the safety of widely used essential drugs, is expected to be set up.
Industry analysts said China's major problems with drug safety are in the areas of anti-infection drugs and traditional Chinese medicine.
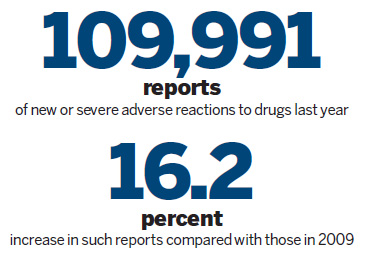
In 2010, China's National Center for Adverse Drug Reaction Monitoring received 692,904 reports of adverse reactions, 8.4 percent more than in 2009, official statistics showed.
Out of that total, 109,991 were reports of new or severe adverse reactions, making for an increase of 16.2 percent year-on-year.
Drug safety incidents have hit the headlines. Hong Kong's Department of Health on Friday ordered the wholesaler of a proprietary Chinese medicine to recall from consumers a batch of Hua Tuo Brand Baoying Dan, known to be used by children for the treatment of generalized discomfort like restlessness at night or fever, Xinhua reported.
The action was called for after a sample was obtained by the department's inspectors and found to contain about two times the permitted level of mercury, it said. The case has been referred to the administration for further investigation.
Strengthening the supervision of essential drugs' production and quality to ensure safety is a working priority for the country's drug authorities, according to the notice.
It also demanded effective measures be adopted to eliminate essential drugs' quality problems and remove any hidden risks.
 0
0 







Go to Forum >>0 Comments